skynesher/E+ by means of Getty Pictures
Thesis
The bears and the bulls each have had their method with the inventory of Cassava Sciences (NASDAQ:SAVA) and most definitely will proceed to take action going ahead. The unique science surrounding its drug candidate is disputed, regardless that in the meantime outdoor establishments and new analysis proceed to verify an obvious drug impact.
The complaint most commonly pertains to the drugâs mechanism of motion, much less to human biomarkers and medical trial effects. I attempt to take a biomarker-based way having a look at many firms within the neurodegenerative and oncology area. To me, Cassavaâs biomarkers appear constant and powerful. I fail to spot how those may well be made up.
The biomarker the medical neighborhood and FDA specifically desire, NfL, has noticed stable discounts in simufilam-treated sufferers. The FDA has granted sped up approval at the foundation of that biomarker in ALS, for the drug Biogenâs (BIIB) Qalsody which resulted in discounts of that biomarker by way of 55% over 28 weeks in comparison to a 12% building up in placebo-treated contributors. Simufilam seems to do the similar in Alzheimerâs sufferers, over a equivalent duration.
Fresh learn about information from Cassavaâs Cognition Repairs Find out about confirms the potential for the full efficacy of simufilam in mild-to-moderate sufferers, the learn about inhabitants of Cassavaâs Segment 3 trials. The knowledge in reasonable sufferers is much less inspiring.
A head-to-head comparability with Leqembi or donanemab could be unimaginable, as each didn’t goal reasonable sufferers, however sufferers in milder illness levels.
Advent
Cassava Sciences is a biotech corporate with one drug in its pipeline, centered â for the instant â at the remedy of Alzheimerâs illness. Its drug simufilam is claimed to bind to misfolded filamin A.
As misfolded filamin A allegedly interacts with amyloid-beta and such interplay would result in inflammatory signaling, a trademark of Alzheimerâs, simufilam would opposite such inflammatory signaling. Simufilam would repair the standard form of filamin A. In doing so, simufilam would result in much less neuroinflammation, any other hallmark of Alzheimerâs, which might result in cognitive growth. I’ve intentionally incorporated a variety of qualifiers within the above rationalization to focus on the extremely disputed science in the back of simufilamâs mechanism of motion which, I imagine, would possibly not but be absolutely understood by way of somebody. Critics of Cassava appear to center of attention principally on that medical phase, alleging that the medical wisdom in the back of simufilam does no longer upload up and that no less than one scientist in the back of the analysis paintings on simufilam didn’t have the desired medical integrity. This dialogue seems to be an everlasting back-and-forth. The stakes also are prime at this level, with 35% of the to be had stocks shorted, and brief presence being systemic since 2021. All sides of the ether right here does no longer develop silent, and I’ve noticed somewhat some inflammatory feedback that experience disincentivized me from offering protection on Cassava Sciences up up to now.
As the present medical center of attention and early successes within the box of neurodegenerative illnesses center of attention increasingly at the aid of inflammatory gliosis and biomarkers, and as I’ve failed to look protection of what I’d believe very important concerns to possible long-term funding or divestments in Cassava Sciences, I sought after to percentage a few of my insights beneath.
Knowledge will make a decision
Cassava Sciences must see topline information from its Segment 3 trials in 2025, that means in a little bit greater than a yr. Those trials have enrolled reasonably rapid, are massive, and feature a length of one year.
Segment 3 evaluate (Company Presentation)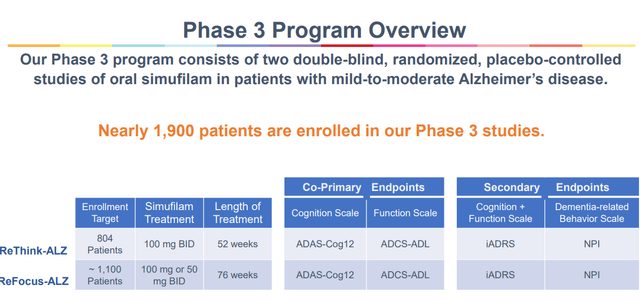
If each are a hit, then Cassava Sciences has two a hit trials and might believe submitting for approval in Alzheimerâs illness.
Those are the consequences from the twelve-month open label learn about.
Abstract open label learn about (Cassava Sciences CTAD presentation)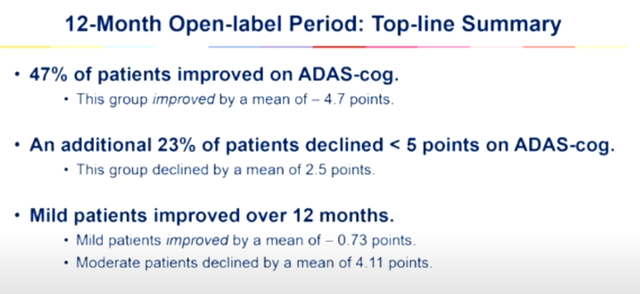
The result of the Cognition Repairs Find out about, which used to be a six-month randomized placebo-controlled trial after sufferers were three hundred and sixty five days on simufilam already, therefore producing information of use as opposed to placebo after an preliminary three hundred and sixty five days at the drug, can then function further evidence of long-term efficacy.
CMS learn about effects (Company Presentation)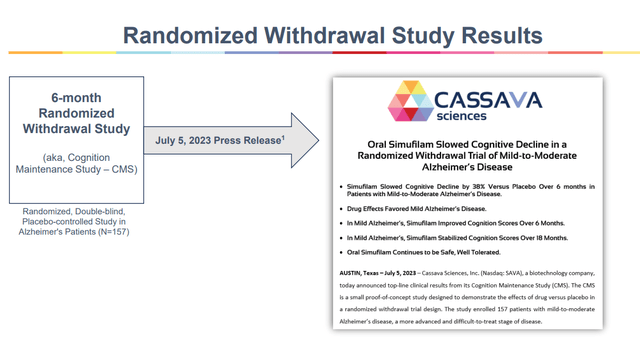
A equivalent impact is noticed within the Cognition Repairs Find out about, the place simufilam slowed cognitive decline by way of 38% as opposed to placebo, with efficacy in light sufferers being more potent.
Adas-Cog11 alternate CMS learn about (CTAD 2023 presentation)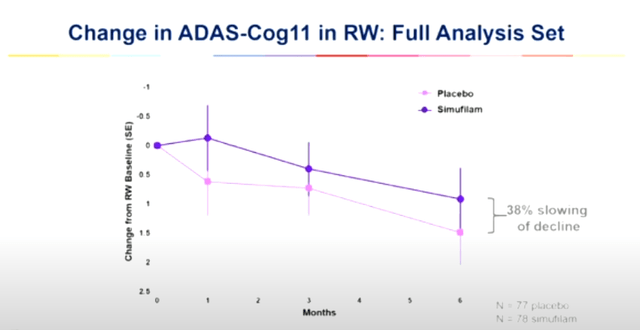
Overall alternate 18 months (CTAD 2023 presentation)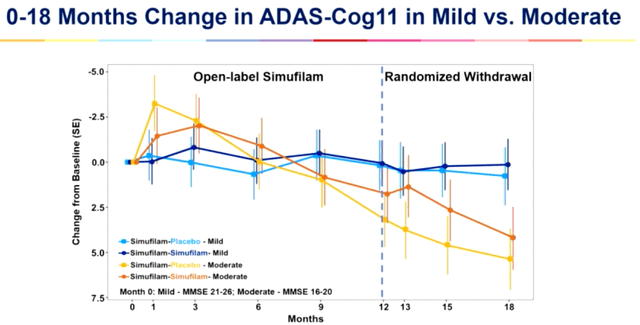
Even though at the moment the knowledge is both non-placebo-controlled or no longer statistically vital, it would outperform Biogenâs Leqembi and Eli Lillyâs (LLY) donanemab if reproduced within the Segment 3 trials. Two arguments might play in desire of higher effects. Sufferers within the CMS learn about had already been on drug for a yr, which might imply most efficacy had already been reached. It seems that, on the time of enrolling within the CMS learn about, some sufferers who were on the reasonable finish of illness within the open label learn about had extra declined to the serious level, which might provide an explanation for the extra fast development of those sufferers. I do observe, alternatively, that the above outcome seems a long way from what had first of all excited the marketplace in February 2021.
Two ongoing Segment 3 trials are ongoing, and each have grow to be the thing of a Citizen Petition asking for they be suspended. The FDA pushed aside that request because it used to be one-sided and because it didn’t go the formal check as as to whether the specific request may well be made in a Citizen Petition. Since that point, 3 camps appear to have arisen; the traders, the non-investors, and people who have made up our minds it’s higher to chorus from making an investment. Even though it had already been denied, the request for trial suspension just lately noticed new sunlight, in mild of the alleged discovery and protection of a draft file of CUNY as as to whether some of the scientists having researched the mechanism of motion of simufilam is chargeable for any wrongdoing. Cassava regarded as that the CUNY file made no findings of knowledge manipulation and referred to a brand new brief assault. CUNY has in the meantime distanced itself from the file. I believe chances are high that small, the FDA if truth be told believes that it âmustâ do anything else at this level. It’s, alternatively, no longer excluded that we see a brand new Citizen Petition from new short-sellers, at the foundation of recent information, which once more would possibly not have the ability to take on the afore-mentioned formal hurdle, however might galvanize a further-declining percentage value. For the report, Cassava Sciences is combating a felony struggle in opposition to some short-sellers.
If the FDA would alternatively halt trials at this level, with a drug confirmed to be protected, exceptional open-label and placebo-controlled effects, testimonials, biomarkers, and powerful make stronger from Cassava shareholders, I imagine it will do one thing totally in opposition to the very thought of medical trials, which might be designed to look whether or not sufferers have the benefit of the drug. If they don’t, the moderately 1,900 sufferers in Segment 3 trials of simufilam might unfortunately sign up for the already considerable collection of sufferers that experience ever been within the considerable failed trials for Alzheimerâs, e.g., anti-amyloid antibodies or BACE inhibitors. This is, alas, the truth of any medical trial; many fail, some prevail, and sufferers are at a loss after they fail. Closing yr, a piece of writing seemed wondering the very science on which those anti-amyloid antibodies are founded. Nevertheless, I’ve no longer noticed a Citizen Petition or different equivalent request, asking for that such trials, regardless that confirmed to be unsafe, be halted.
Additionally, anti-amyloid antibodies are identified to be unsafe, opposite to simufilam. An unsafe remedy must be a pink flag, which many physicians and several other Advisory Committee individuals have voiced when Aduhelm used to be about to obtain sped up approval at the foundation of aid of amyloid beta as a surrogate endpoint.
The biomarkers
Advent
As Alzheimerâs is a human illness, it will make sense that medical information in people is what the FDA will have a look at maximum after all. Even though one can simplest wager at this level what Cassavaâs Segment 3 trials will file, one can in the meantime take a look on the biomarkers that experience already been reported by way of Cassava Sciences.
That’s what I have a look at most commonly all the way through my paintings, and which is the root for my stable conviction in INmune Bio. In my protection of Annovis, which I’m extra skeptical about, I had made a comparability with information on other biomarkers reported by way of each firms. I copied some right here.
Related biomarkers Annovis – Cassava Sciences (28 days) (Personal Paintings)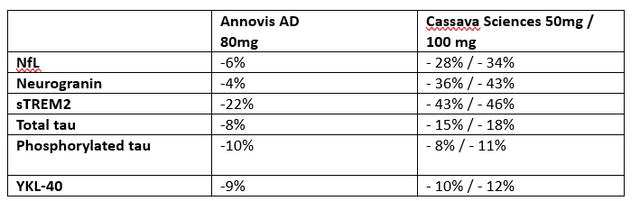
What’s hanging this is the consistency of knowledge coming from Cassava Sciencesâ simufilam. On reasonable, Cassavaâs effects seem about 3-4 occasions as stable as the ones of Annovis, and whatâs extra, Cassavaâs biomarkers hugely progressed on the six-month time level. If we come with the ones, we get the next.
Related biomarkers Annovis – Cassava Sciences (Personal Paintings)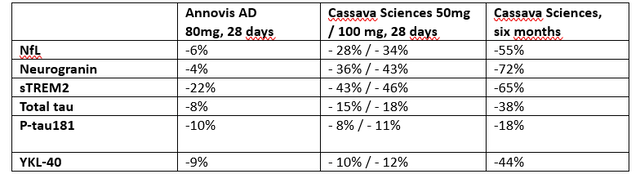
For the report, those aren’t all biomarkers reported by way of Cassava Sciences, however those who I regarded as are compatible for comparability with any other drug candidate and that have been to be had all the time issues.
Biomarkers of neuroinflammation/glial task
YKL-40 is a well-established biomarker of neuroinflammation. In Alzheimerâs illness, its expression is steadily noticed in astrocytes, the mindâs maximum considerable immune cells or glial cells, extra specifically in clusters round amyloid plaques. The inflammatory gliosis stated above refers to a prolonged state of reasonable irritation produced by way of those glial cells, together with microglia. The speculation in the back of anti inflammatory remedies for Alzheimerâs is that by way of lowering their inflammatory phenotype, they can revert to their unique serve as, which is caring for the mind, a serve as that they have got given up by way of final in an inflammatory state for too lengthy.
Any other marker of the reactive and pro-inflammatory state of glial cells is sTREM2, a marker specifically of microgliosis. Microglia are much less considerable than astrocytes however had been recognized as similarly necessary within the architectural processes of the mind.
The aid by way of simufilam of each YKL-40 and sTREM2 by way of 44% and 65%, respectively over the process six months, signifies to me {that a} very stable anti inflammatory reaction is being generated by way of simufilam. I’d no longer know the way those biomarkers may well be indicative of placebo-like efficacy, moderately on the contrary, they end up that simufilam has an impact on a mobile stage.
In that appreciate, as TREM2 is a very powerful genetic chance issue for Alzheimerâs illness, Alector (ALEC) has a monoclonal antibody in trials concentrated on TREM2 on microglia in Alzheimerâs and AbbVie has been given an way to expand and commercialize AL002, for milestones probably totaling $487.5 million.
However sTREM2 and YKL-40 aren’t any markers of neurodegeneration.
Biomarkers of neurodegeneration
Overall tau and p-tau181 may well be noticed corresponding to neurofibrillary tangles of tau protein demonstrating lack of construction in tiny vessels throughout the neurons. Neurogranin may be a marker of neurodegeneration, however no longer one this is as established as NfL.
That brings me to NfL. NfL is a biomarker that are supposed to have the ability to have price throughout neurodegenerative illnesses, each as a measure of the rate of neurodegeneration in addition to a measure of regeneration, remedy impact in different phrases. In MS, ALS, and AD, the medical neighborhood seems to align on its prognostic price, additionally in AD in Down syndrome, Creutzfeldt-Jakobâs illness, and FTLD. Greater NfL ranges in AD had been related with neuronal loss of life and axonal degeneration. Its correlation with cognitive decline has been established greater than as soon as additionally outdoor of Alzheimerâs illness. That is hanging as one would believe hallmarks of illness, e.g. amyloid aggregates and neurofibrillary tau tangles, as maximum predictive of illness evolution or remedy impact. This is, alternatively, no longer the case.
NfL, to me, is by way of a long way a very powerful biomarker reported by way of Cassava Sciences, because it has given technique to sped up approval of Biogenâs tofersen/Qalsody [FDA slideshow, meeting transcript, briefing document, approval PR].
NfL slide briefing FDA for tofersen/Qalsody approval (FDA)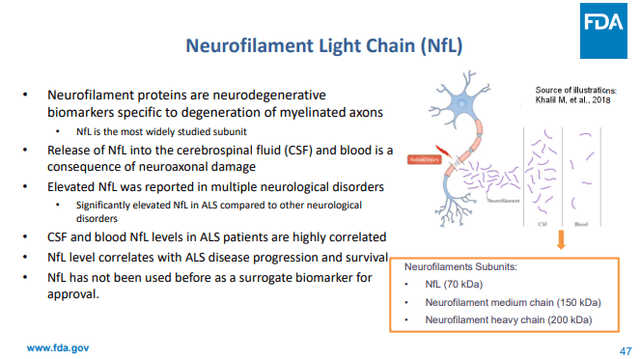
Tofersenâs trial effects didn’t succeed in the trialâs number one endpoint, alternatively, remedy with Tofersen confirmed a 55% lower in NfL values over 28 weeks, all whilst sufferers on placebo noticed an building up of 12%. The sped up approval used to be preceded by way of a unanimous vote by way of the Advisory Committee bearing in mind that, despite the trialâs failure, as a surrogate endpoint plasma NfL may just fairly most probably are expecting medical get advantages in SOD1-ALS. Issues of the Advisory Committee were, amongst others, the FDAâs personal detailed research together with to be had literature and knowledge offering sufficient proof to make stronger NfL as a surrogate endpoint, and the concept that delaying acclaim for a sign with such prime unmet want could also be unethical.
Tofersen lowered NfL values in ALS by way of 55% over 28 weeks. Simufilam does precisely the similar over a equivalent time frame, a 55% lower over six months. I’m seeing some possible caveats. The indication isn’t similar, with NfL ranges being specifically increased in ALS. There’s a risk that those biomarker effects originate from sufferers in milder levels of illness, because it had seemed that the preliminary effects posted by way of Cassava Sciences had come most commonly from sufferers at milder levels of AD. Those information are generated from a small and extra specifically open label learn about and therefore would possibly not result in sped up approval as such, however it’s arduous to believe the values of 25 sufferers could be misguided or manipulated. If sufferers on placebo would have a 55% lower in that biomarker, Cassavaâs information are stable, however they don’t seem to be the most productive ever reported, which might validate them. Aside from equivalent numbers from Tofersen in ALS, INmune Bioâs (INMB) Xpro confirmed an 84% aid of this biomarker over the path of 3 months in AD sufferers. For extra reference, Alectorâs AL001, concentrated on the progranulin gene for frontotemporal dementia, noticed a 27% aid over the path of seven months. Biogenâs Leqembi noticed aid of biomarkers of irritation however didn’t see aid of NfL. Knowledge on Eli Lillyâs donanemab must nonetheless be revealed, I imagine. In a failed trial, Rocheâs (OTCQX:RHHBF, OTCQX:RHHBY, OTCPK:RHHVF) gantenerumab slowed NfLâs worsening, with handled sufferers seeing that biomarker move up 1.7% in comparison to 3.4% for placebo. Those are higher trials and it says one thing concerning the customary evolution of NfL as a biomarker.
BioVie (BIVI) must file NfL information in January 2023, after its topline Segment 3 readout in a few monthâs time. For Anavex (AVXL), greater than a yr after my protection during which I had stated that I’d stay up for biomarker information to believe my ultimate place at the corporate, I’m nonetheless looking forward to all of the biomarker readout which must come with NfL information.
Complete approval / sped up approval
Cassava Sciences is recently on a trail in opposition to conventional complete acclaim for Alzheimerâs illness.
Because it now seems that simufilam might carry out higher in sufferers with light AD and much less just right â or no longer – in sufferers with reasonable AD, I believe it conceivable however no longer sure whether or not the ones trials will achieve achieving statistical importance. My take is that Cassavaâs possibilities of luck are upper than the ones of failure, however the reasonable crew might pull down the full outcome. If Cassava Sciences had identified this prematurely, in all probability the easier selection would had been to start out trials in MCI and gentle AD, similar to large pharma maximum steadily does it, as those sufferers appear much more likely to have a remedy impact. I once more repeat any other level I’ve made sooner than on many different firms, and that’s â for Cassava Sciences – that the degrees of misfolded filamin A within the AD inhabitants aren’t but nicely sufficient established in literature, and that subsequently it’s not excluded that sufferers with out such sufficiently increased ranges would possibly not sufficiently have the benefit of simufilam.
Cassavaâs contemporary presentation at CTAD confirmed that some sufferers who had been regarded as reasonable had been if truth be told already on the serious levels of illness, which might play in desire of Cassavaâs Segment 3 trials. On the other hand, Cassava can have a whole lot of biomarker information from that Segment 3 trial, which must come with NfL information. If this is the case, in case Cassava e.g. sees stable efficacy in light sufferers which seems most probably, the corporate might attempt to opt for sped up approval at the foundation of the exceptionally stable biomarker information, specifically NfL. A confirmatory trial might in such case want to be run, e.g. in light sufferers by myself, if already each Segment 3 trials wouldnât have the ability to sufficiently make that difference by way of themselves.
The above does no longer quilt all possible techniques towards approval. I’m conscious supporters of Cassava Sciences had been in desire of Cassava Sciences being granted Step forward Treatment Designation.
Outdoor affirmation of simufilamâs impact
It now seems that 4 instructional establishments have generated information in make stronger of the organic task of simufilam. This features a Parisian lab confirming that simufilam potently lowered amyloid β binding to α7nAChR, with a unique method than were used sooner than. That turns out to align with simufilamâs proposed MoA as proven beneath:
Simufilam MoA (Company Presentation)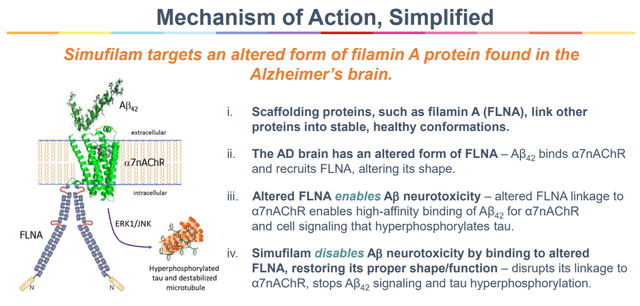
The latter analysis additionally comprises this word:
Simufilam at 100 fM, 10 pM or 1 nM lowered the discharge of inflammatory cytokines tumor necrosis issue α (TNFα), interleukin (IL)-6 and IL-1β by way of roughly 75% or extra (p < 0.001; Determine 3).
For me, this sentence, even though it comes from preclinical paintings, is a sturdy validator of simufilamâs possible efficacy. As famous in previous protection of various firms within the area by way of myself and in two and probably extra articles by way of In search of Alpha analyst Imaginative and prescient and Worth underneath the not unusual identify âCapitalizing On Irritation’s Position In Neurological Illnessâ, tackling irritation whilst taking into consideration homeostasis could also be very important to treating neurodegenerative illnesses. Each BioVie (BIVI) and INmune Bio (INMB) have correlated aid of TNF with cognitive efficacy, and this builds on a number of meta-analyses appearing that non-selective and non-brain-penetrant TNF inhibitors result in lowered chance of Alzheimerâs illness. A discount of the above cytokines by way of 75% isn’t not anything, however I do observe one caveat; complete TNF signaling must no longer be inhibited to permit for homeostasis; one will need the microglia and astrocytes to accomplish their nurturing and brain-architectural purposes. INmune Bioâs Xpro selectively objectives soluble TNF. I’ve no longer noticed analysis on simufilamâs functions on this appreciate, however I’m prepared to suppose they could also be generated at some point, seeing a reputedly lasting impact no less than in milder sufferers.
Outdoor validation is a sturdy component enjoying in desire of simufilamâs efficacy. In mild of this, regardless that some have regarded as it to be needless to verify protection of a drug that has placebo-like efficacy – it sort of feels reliable to me that Cassava confirms that simufilam is protected.
Some critics have when put next Cassava Sciences to Theranos, however insofar as I do know, Theranosâ claims had been by no means validated by way of outdoor events. To correctly dispute simufilamâs results, I imagine one must invalidate those outdoor validations. Insofar as I’m conscious, no outdoor paper has been retracted or has been underneath scrutiny. I’m subsequently susceptible, once more, to believe simufilam as a possible remedy for Alzheimerâs illness.
Budget
As of June 30, 2023, Cassava Sciences had $168.4 million in money and money equivalents. Cassava Sciences has a money burn price of about $66 million in keeping with yr, which must permit it to finalize its Segment 3 trials and finance extra analysis.
Dangers
On all sides of the location of Cassava Sciences, I’ve noticed strong-minded traders. That can grow to be a chance when each and every particular person with a possible counterargument might grow to be categorised as professional or in opposition to.
I’ve quoted trial design dangers and dangers associated with the loss of stable medical background on filamin Aâs involvement in Alzheimerâs illness, and if this is the case, to what extent and in what number of sufferers.
Additional brief involvement and allegations are conceivable, as Cassava Sciences is the thing of continuing debate amongst traders.
In spite of everything, at any time, dangers and possible advantages of an funding want to be open for reassessment, specifically as tendencies within the pharmaceutical trade can alternate unexpectedly. Within the coming yr, such tendencies may just amongst others come from BioVie (BIVI), Annovis (ANVS), Alzheon [private], and Athira Pharma (ATHA).
My place
Presently, I’ve no longer taken an funding in Cassava Sciences, most commonly as a result of the unpredictability of no matter subsequent motion shall be taken by way of both sides. Within the present case of Cassava Sciences, my biomarker-based way has little predictive price for the inventoryâs strikes, so I wish to put money into probably overpassed shares with equivalent possible.
The afore-mentioned concerns do indicate my bullishness on simufilamâs possible, and save for extra surprising occasions which might regulate my concepts, I plan to take an funding within the inventory previous to the Segment 3 readout if at the moment the marketplace cap would nonetheless be such that vital upside is conceivable in case of luck. This is, alternatively, a very long time out, and with the statistical possibilities of 2024 turning out to be bearish being low, I will be able to have to look how issues play out.
It’s for the above causes that I give Cassava Sciences a Bullish ranking, extra for the potential for its drug candidate than for the unpredictability of its inventory actions.
Conclusion
For a very long time at this level, my bullishness on simufilam as a drug candidate for Alzheimerâs illness has been according to biomarker information, most commonly as a result of the 55% aid of NfL values over the process six months. The opposite biomarkers reported by way of Cassava Sciences seem to align with this reporting, which might provide an explanation for why Cassava Sciences has reported just right cognitive information in sufferers with light Alzheimerâs illness. Additional outdoor confirmations of simufilamâs efficacy, together with a Parisian lab that still noticed large discounts in primary pro-inflammatory cytokines, verify that stance.
The inventoryâs volatility and unpredictable brief movements are a priority, and a good research had to come with caveats, for instance in regards to the Segment 3 trials which come with reasonable sufferers and the unknown share of Alzheimerâs sufferers who’ve sufficiently increased ranges of misfolded filamin A to answer remedy.
If simufilam, proven to be protected, may just reproduce equivalent effects as the ones noticed sooner than, this time in Segment 3 trials and with statistical importance, then I’d be in desire of it having its position in the marketplace at some point. If an efficacy sign would simplest be detected in light sufferers, then the NfL information may just permit for an sped up way plus confirmatory learn about very similar to how Biogenâs Qalsody used to be licensed.
For the explanations stated above, I give Cassava Sciences a bullish ranking.